Preimplantation Genetic Testing (PGT) is performed by sampling the developing embryo at the appropriate time point(s) during an In Vitro Fertilization (IVF) cycle.
When and How Do We Get Started?
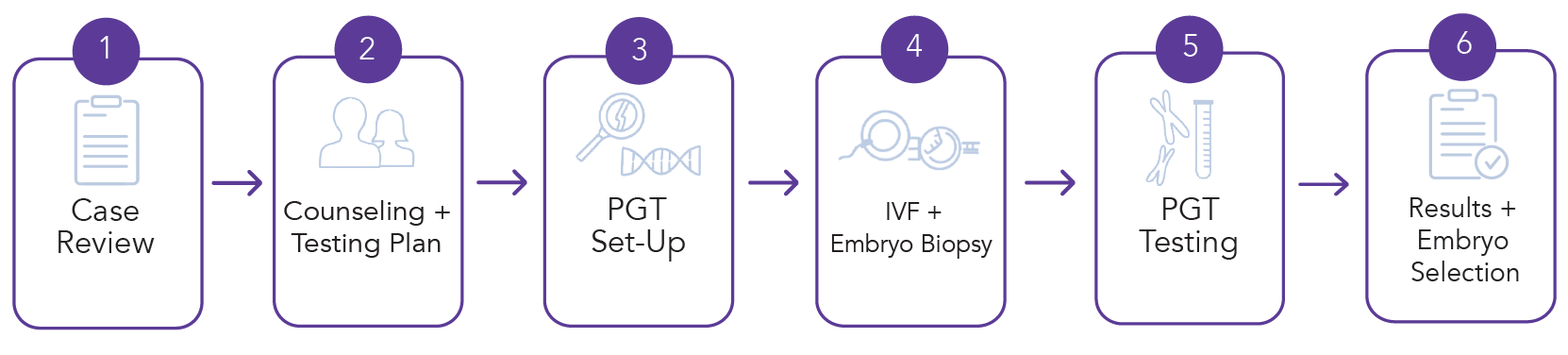
In order for case review to begin, RGI will need to receive a test requisition form from a healthcare provider along with any genetic reports, if applicable. The patient should NOT start IVF stimulation medications until the PGT setup is complete. RGI will contact the IVF center (and the patient) once the PGT setup is complete. Setup cannot be started until completion of patient counseling, processing of necessary paperwork (such as a HIPAA form, consent documents, financial agreements/clearance, and other paperwork) as well as receipt of necessary DNA samples. Setup and DNA samples are not necessary for aneuploidy testing (PGT-A). We ask that the IVF center and patients keep RGI informed of important dates such as medication start dates, stimulation start dates and hCG administration.
PGT results are typically ready within 7 to 10 business days. Once results are understood, the process of embryo selection can determine which are most suitable for transfer. Embryos remain cryo-preserved (frozen) and available for future embryo transfer.
Discover the best chance for a healthy pregnancy and peace of mind with RGI.
How is PGT performed?
PGT is preformed on biopsy samples obtained from a blastocyst embryo.
Reproductive Genetic Innovations has been performing PGT-M since its inception in 1989, and remains one of the most active centers offering preimplantation genetic testing in the world. Our scientists were the first to develop a strategy for preimplantation genetic testing for single genes that is optimized for HLA matching. RGI also offers testing for all 24 chromosomes, using Next-Generation Sequencing.